Explain Modern Periodic Law And Long Form Of Periodic Table
One taken by me and one from the company. Metalloids are found in a zig-zag manner between the metals and the non-metals.
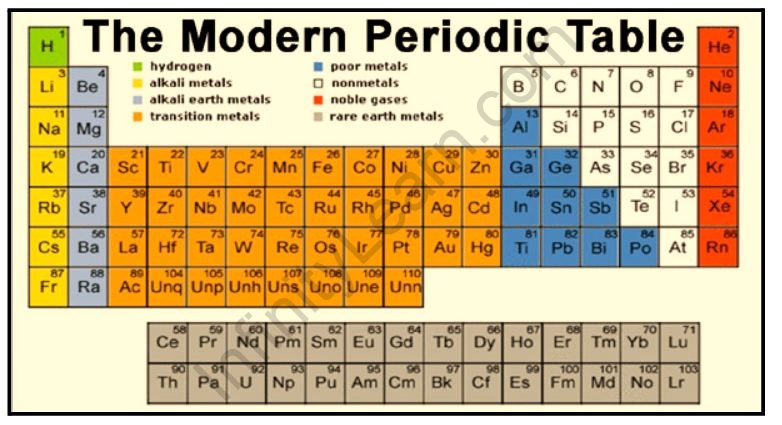
Long Form Of The Periodic Table Infinity Learn
Draw its electronic dot structure.

Explain modern periodic law and long form of periodic table. E Name the scientist who prepared modern periodic table. A The modern periodic law states that the properties of elements are a periodic fu nction of their atomic numbers. Some forms emphasise chemical reactions and valence whereas others stress the electronic configuration of elements.
My pictures or their pictures. Non-metals are found on the right side of the Modern Periodic Table. Write the formula of the product formed when the element A atomic number 19 combines with the element B atomic number 17.
The horizontal rows. Long form Periodic Table In the long form each period correlates to the building up of electronic shell. A State modern periodic law.
32 is the most convenient and widely used. A State the modern Periodic table law. A Elements in modern periodic table are arranged on the basis of increasing atomic masses.
His first Periodic Table was compiled on the basis of arranging the elements in ascending order of atomic weight and grouping them by similarity of properties. The modern statement of this relationship the periodic law is as follows. In Mendeleev s Periodic Table the elements were arranged in the incr easing order of their atomic masses.
The properties of the elements are periodic functions of their atomic numbers. B How many periods and groups are there in the modern periodic table. B What are the advantages of the long form of the periodic table over Mendeleevs periodic table.
This table is based on Mendeleevs periodic table and the periodic law. To Learn about the modern periodic table of elements and its significance and also learn about the manner in which the elements are classified across the modern periodic table Visit BYJUS for more information. Explain by giving reason.
Metals are found on the left side and centre of the Modern Periodic Table. Modern Periodic Law states that the properties of elements are a periodic function of the atomic number Metals are found on the left side and centre of the Modern Periodic Table. Iii State modern periodic law.
A According to modern periodic law physical and chemical properties of elements are periodic function of their atomic numbers. A modern version the so-called long form of the Periodic Table of the elements Fig. However cobalt with atomic mass of 5893 amu was placed before nickel having an atomic mass of 5871 amu.
The picture on the left was taken by me. You can see photographs of all the samples displayed in a periodic table format. Jensen of the University of Cincinnati.
The first two groups 1-2 s-block and the last 6 groups 13-18 p-block make up the main-group elements and the groups 3-12 in between the s and p blocks are. B When elements are arranged according to increasing atomic numbers there is a periodicity in the electronic configurations of the elements. I have two photographs of each sample from the set.
C In modern periodic table the element nickel of lower atomic mass is kept before the element cobalt of higher atomic mass. Mendeleevs law allowed him to build up a systematic periodic table of all the 66 elements then known based on atomic mass which he published in Principles of Chemistry in 1869. A The modern periodic table states that Physical and chemical properties of elements are a periodic function of their atomic numbers.
B Elements in Mendeleevs periodic table are arranged on the basis of increasing atomic numbers. Or you can see both side-by-side with bigger pictures in numerical order. Give reason for the same.
Modern periodic Law. Numerous forms of Periodic Table have been devised from time to time. The same virtue is also seen in a version of the periodic table shaped as a pyramid a form suggested on many occasions but most recently refined by William B.
Non-metals are found on the right side of the Modern Periodic Table. A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column Figure 2. Hydrogen occupies a unique position in Modern Periodic Table.
Metalloids are found in a zig-zag manner between the metals and the non-metals. The properties of elements are a periodic function of their atomic number. Modern Periodic Table of Elements - Modern Periodic suggest that Physical and chemical properties of the elements are the periodic functions of their atomic numbers.
Modern periodic law The physical and chemical properties are a periodic function of their atomic number.

Modern Periodic Table Law Features Classification Advantages Positions

Arrangements In Modern Periodic Table Long Form Of Periodic Table Freakgenie

Chemical Periodicity And The Periodic Table The Modern Periodic Table Download Scientific Diagram
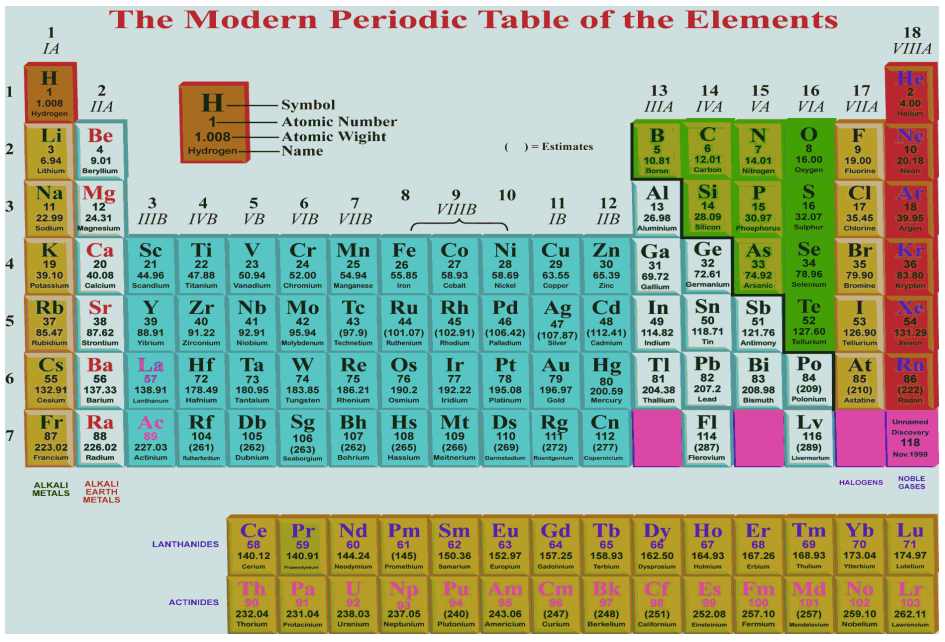
Modern Periodic Table And Its Significance A Plus Topper

Modern Periodic Table Learn Everything About Periodic Table
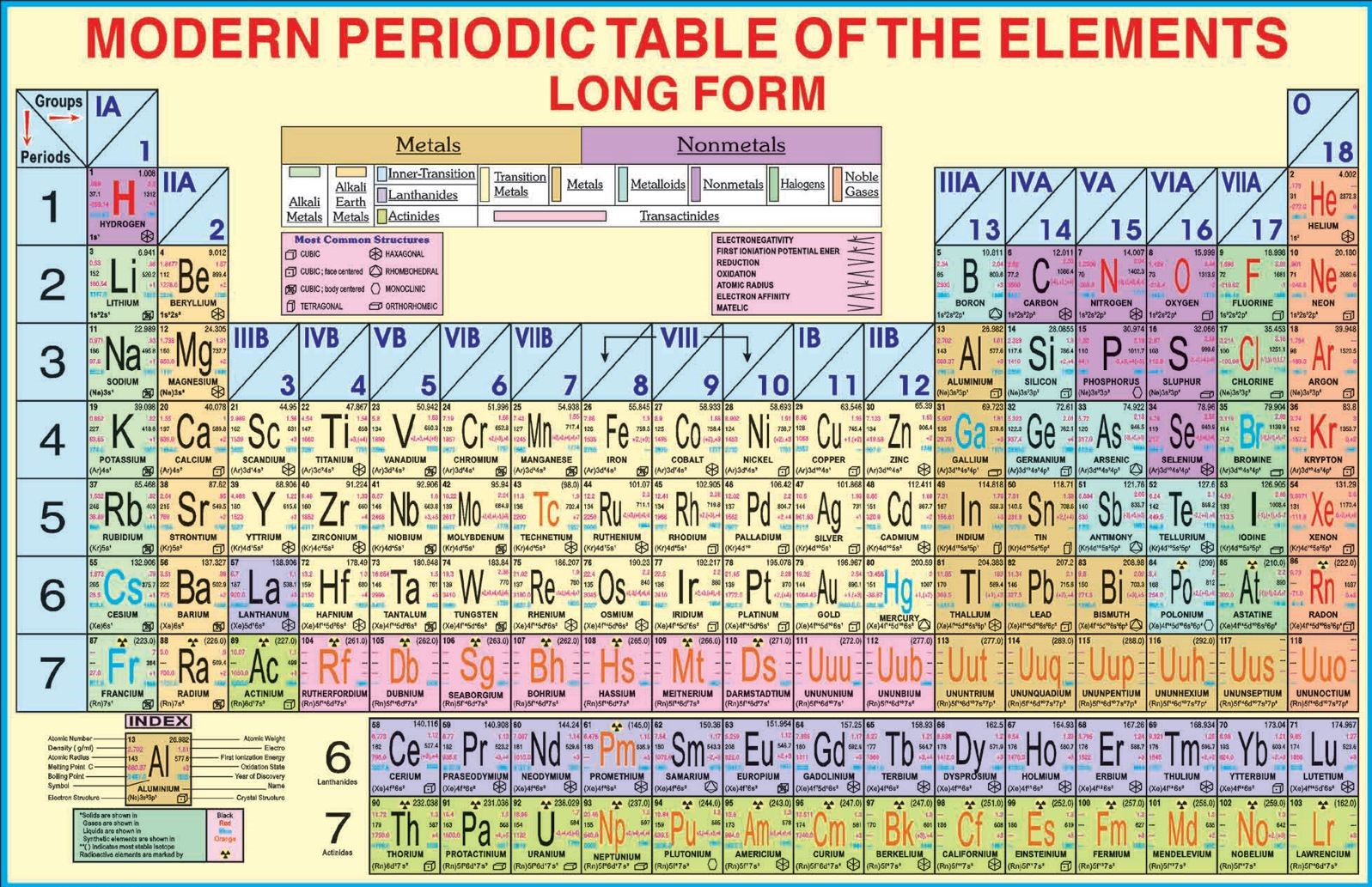
Classification Of Elements Modern Periodic Table Online Science Notes
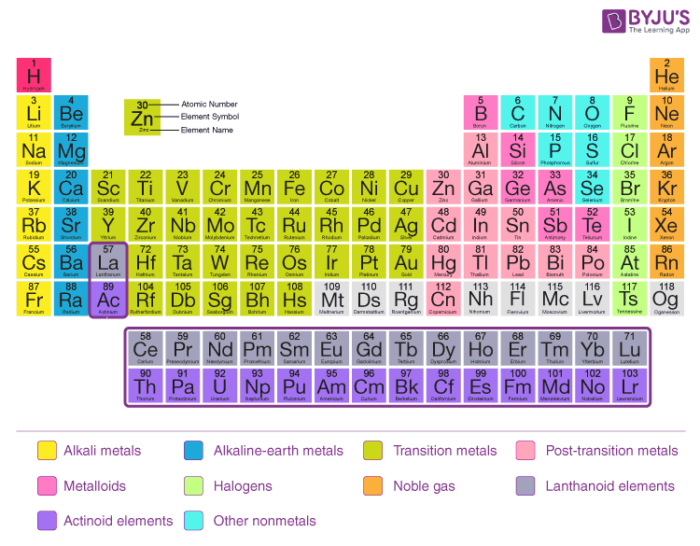
Modern Periodic Law With Detailed Periodic Classification Of Elements
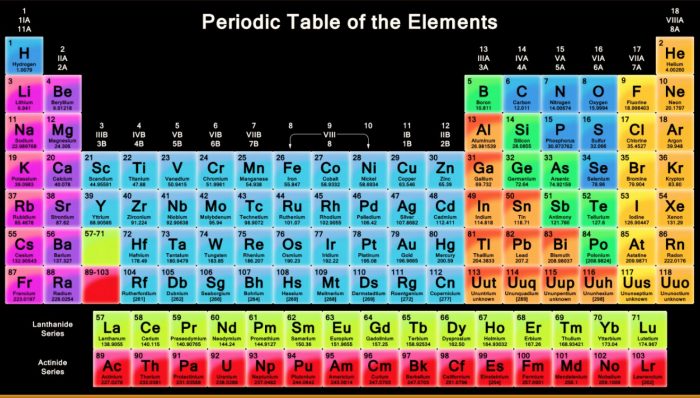
Modern Periodic Table Class 10 Periodic Classification Of Elements

Belum ada Komentar untuk "Explain Modern Periodic Law And Long Form Of Periodic Table"
Posting Komentar